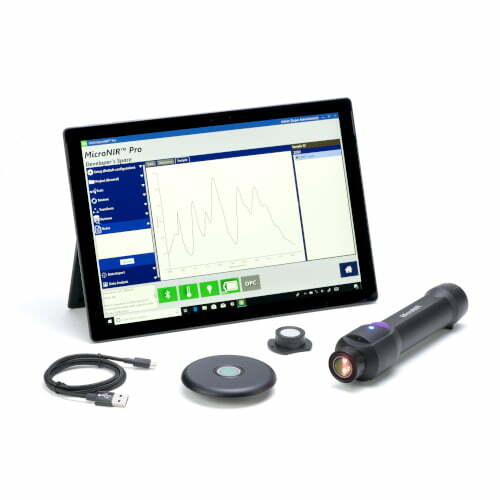
cGMP Raw Material Verification – Progeny by Rigaku
With the push towards 100% raw material inspection, Progeny enables fast and simple PASS/FAIL results. APIs and Excipients are analysed in seconds without opening packaging, thereby never exposing personnel or product to contamination.
Progeny is our latest generation handheld Raman spectrometer, specifically designed for regulated Pharmaceutical and Life Science industries. Easy to use by QC or non-QC staff for enhanced lean manufacturing quality assurance, quality control, counterfeit pharmaceutical screening, and other applications.
Data Integrity, Electronic Signature review/approval, full 21 CFR 11 compliance plus simple validation work-flow are just some of the features of Progeny. Antech have over 100 handheld instruments installed for RMID within regulated sites across Ireland. We have the expertise to answer all your validation and regulatory queries. Contact us today for more information.
>> ProgenySync Software << is available now!
Lean cGMP Raw Material Verification
- Little to no sample preparation
- Non-destructive analysis
- Analyse without opening packaging
- No chemical consumables or disposal costs
The globalisation of the pharmaceutical supply chain and push towards 100% material inspection has led to a significant increase in the demand for cost effective, efficient and regulatory compliant methods for raw material identification (RMID) and chemical verification. Strict quality assurance and quality control measures are more important than ever to ensuring the safety and efficacy of products before reaching consumers.
Traditional workflows involve sending samples to external laboratories for analysis. However, this can result in additional costs and time delays. By implementing a raw material identification method that offers ease of use, a comprehensive range of sampling capabilities and rapid, customisable software at the point of need, a lean manufacturing process can be achieved.
Pharmaceutical manufacturers can now achieve 100% raw material identification, verify chemicals and solvents, as well as authenticate final products quickly and confidently. Using Progeny 1064nm handheld Raman, users can achieve Good Manufacturing Practices and comply with 21 CFR Part 11.
Raman spectroscopy is now recognised by the U.S. Pharmacopoeia and the European Pharmacopeia for raw material identification. Progeny Raman is an FDA 1040, USP, EP, MHRA, HPRA accepted technique and our team is available to address any inquiries or provide our Progeny Statements of Regulatory Compliance upon request.
Progeny Raw Material Identification Solution
Progeny 1064nm handheld Raman spectrometer broadens the analysis range for pharmaceutical, biopharmaceutical, nutraceutical, and cosmetic raw materials; enabling manufacturers to achieve positive material identification outside the lab, inside the lab or anywhere rapid lab-quality analysis is needed. Progeny offers unique benefits that until now have not been delivered:
- Eliminates sample interference – fluorescence or moisture
- User adjustable focus ensures materials’ analysis, not the packaging
- User customisable analysis settings
- Truly compliant 21 CFR Part 11 digital signatures
Ergonomics
Flexibility for use throughout the manufacturing process and beyond. Materials’ analysis using one device on the warehouse dock, in the holding area or clean room, within the laboratory, at-line, post process, pre-dispensing from the warehouse or in the field is now a reality. Progeny features include a smart-phone inspired user interface, with analysis initiation and navigation via the touchscreen display or large buttons. The sleek design delivers truly one-handed operation. Tested and certified rugged to military MIL-STD 810G & IP-68 requirements.
Progeny successfully overcomes sample-induced fluorescence interference with the use of a unique 1064nm excitation laser for expanded positive material identification. Varying moisture content and particle size is not an issue either. Progeny handheld Raman spectrometer delivers the industry’s widest and most comprehensive range of raw material identification and finished pharmaceutical product authentication in a handheld, dedicated for GMP sealed platform.
1. Faster scanning speeds for all materials
2. Reduced fluorescence: 1064nm laser allows analysis of a much broader range of materials including, coloured materials (and coloured packaging), Polysorbates, cell cultures plus much much more
3. E-signature: Review, Approve, Reject and sign electronically with full 21 CFR Part 11 compliance.
4. Dual-purpose docking station:
a. provides seamless data transfer to your network, LIMS or other data storage location
b. transforms the handheld device into a benchtop spectrometer
c. charger function allows for uninterrupted operation
5. WiFi or USB connectivity
6. Smart phone inspired touch screen interface
7. Integrated digital camera and barcode reader
8. MCC, Hypromellose, Croscaramellose, Hydroxypropyl Methylcellulose and other cellulose materials are now possible to identify
9. Calcium Hydrogen Phosphate possible
10. Improved data integrity and audit trails
11. Fully ruggedised and certified to military MIL-STD-810G plus IP-68 standards
12. Batch reports generated automatically
13. No moving parts or consumables
14. Dedicated SOP Word templates for method creation and validation
15. Improved cGMP compliance
16. Integrated software providing advanced analysis features that streamline processes
• Customisable settings
• User permission levels
• Batch mode
• Multiple scans per container e.g. top, middle and bottom of a single barrel
• Transfer data seamlessly and securely
Advanced Performance
- 1064nm laser excitation to minimize fluorescence interference
- Spectral resolution reveals minor differences in similar material
- Spectral range 200-2500cm-1 covers the most relevant molecular spectral features
- Transmissive volume phase grating design for years of alignment-free operation
- TE cooled InGaAS 512 pixel detector for high resolution results
User Control
- 30-490 mW adjustable laser power in 5 mW increments to optimize analysis
- 5ms to 30s adjustable exposure time in 5ms increments for more accurate results
- Adjustable focus nose cone to ensure analysis of materials through packaging
CompleteID Software
- Administrator created workflow methods specific to material ensures accurate analysis
- Rapid addition of new materials to your custom on-board library
- Truly 21 CFR Part 11 compliant digital signatures
- On-board barcode scanner for error-free sample information entry
Sophisticated Ergonomics
- Optimal for one-handed operation:
29.9cm x 8.1cm x 7.4cm (11.8in x 3.2in x 2.9in) and weighing ~1.5kg (3.5lbs) - IP-68 rated ruggedised aluminium housing for maximum durability and minimisation of cross-contamination
- Large high resolution screen allows for high visibility in confined spaces and outdoors
- Choice of user interface:
Smartphone-inspired touchscreen provides fast learning-curve
Softkey buttons for one-handed operation while wearing protective gloves
Unique Accessories
- Docking station for easy data transfer to LIMS or Server
- Adjustable focus vial adapter to accommodate your different vial sizes
- Tablet adapter to accommodate different sized tablets and other sample types for increased flexibility
Additional Services
- IQOQPQ document and on-site validation services
- Performance qualification and recertification services
- Standard Operating Procedure templates in MS Word
Other Specifications
- Certifications: FDA 1040, 21CFR11, CE, ISO 9001:2008 Certified Manufacturing facility
- External battery charger: 100~240VAC
- Operating temperature of 20 to 50degC
Learn more about how Progeny is different
Easily identify mislabeled drums of raw materials or suspected medications.
Click Here to download the Progeny Raman Brochure
Click Here to download the Progeny Raman Spec Sheet
Click Here to download White Paper: Advantages of 1064nm Handheld Raman
Click Here to download Application Note: Identification of Excipients using Progeny Raman
Click Here to download Application Note: Polymorph Monitoring using Progeny Raman
Click Here to download Application Note: Counterfeit Medicine Detection using Progeny Raman
Click Here to download Application Note: Cell Culture Media ID in Biopharma using Progeny Raman
Click Here to download Application Note: Drug product ID using Progeny Raman
Click Here to download Application Note: Analysis of Dietary Supplements using Progeny Raman
Click Here to download Application Note: Identification of Polysorbates using Progeny Raman
Related Products

Newsletter Signup
Sign up to our newsletter for the most up-to-date development of products, industry news, special offers and much more!
Location: IRELAND
Unit 21, Waterford Business Park,
Cork Road, Waterford
Ireland, X91 P224
E-mail:
Contact: UK
Tel: +44 (0)20 346 898 13
E-mail: info@antechsolutions.co.uk
Web: www.antechsolutions.co.uk